The pharmaceutical industry faces unique technology challenges that can make or break drug development timelines and regulatory compliance. Companies need specialized IT solutions for pharmaceutical industry operations that go far beyond standard business systems.
At Clouddle, we understand how the right technology infrastructure directly impacts research outcomes, manufacturing efficiency, and patient safety. The stakes are too high for generic IT approaches.
What IT Infrastructure Do Pharmaceutical Companies Actually Need?
Network Architecture That Handles Complex Data Flows
Pharmaceutical companies process massive datasets daily, from clinical trial information to molecular modeling computations. Your network infrastructure must support data transfers measured in terabytes, not gigabytes. Companies like AstraZeneca handle over 170 million rows of manufacturing data daily, which requires network speeds that can process this volume without bottlenecks.
Multi-site operations need dedicated fiber connections with minimum 10 Gbps bandwidth between research facilities, manufacturing plants, and headquarters. Standard business internet cannot handle the computational demands of drug discovery algorithms or real-time quality control systems. The infrastructure must scale to accommodate peak processing loads during clinical data analysis phases.
Compliance-First Security Architecture
FDA 21 CFR Part 11 requirements demand that every system modification creates an audit trail with electronic signatures and timestamps. Your infrastructure needs built-in compliance systems that capture every data access, modification, and transfer automatically. HIPAA compliance adds another layer, which requires encrypted data storage and transmission with role-based access controls that restrict information based on job functions.
Healthcare organizations experienced 444 reported incidents in 2024, comprised of 238 ransomware threats and 206 data breach incidents. This reality makes zero-trust network architecture essential for protection. Implement network segmentation that isolates research data from manufacturing systems, with separate authentication protocols for each environment.

Cloud Strategy for Research Acceleration
Research and development operations benefit dramatically from cloud processing power and storage flexibility. AstraZeneca plans to analyze two million genomes through cloud solutions, which will conduct 51 billion statistical tests in under 24 hours. Your cloud strategy should prioritize hybrid deployments that keep sensitive intellectual property on-premises while public cloud resources handle computational analysis.
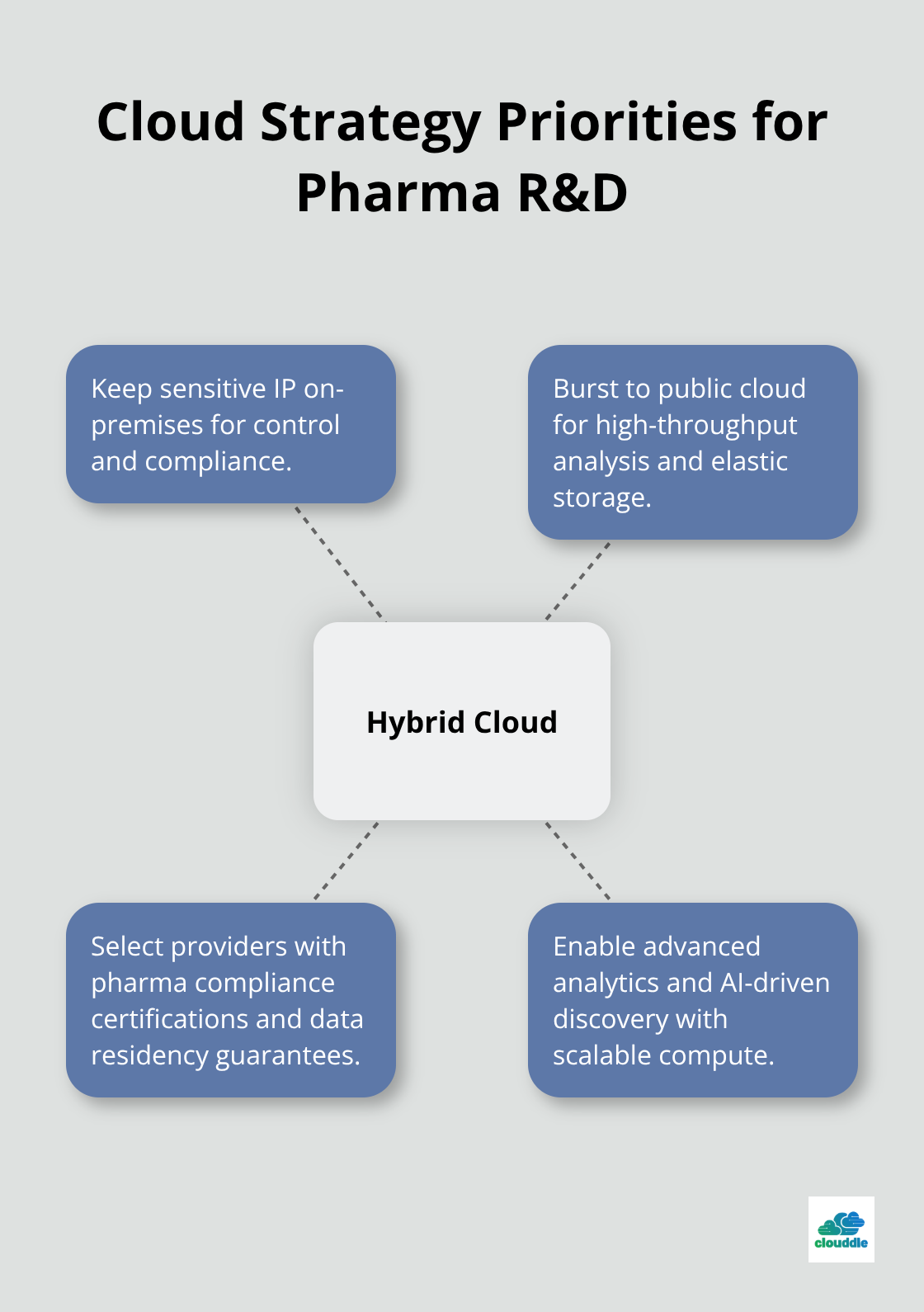
Choose cloud providers with specific pharmaceutical compliance certifications and data residency guarantees that meet international regulatory requirements. The right cloud architecture becomes the foundation for advanced analytics and AI-powered drug discovery platforms that pharmaceutical companies increasingly rely on for competitive advantage.
Which Systems Drive Pharmaceutical Production Excellence
Laboratory Information Management Systems That Actually Work
Laboratory Information Management Systems form the backbone of pharmaceutical research operations, but most companies choose platforms that create more problems than they solve. Your LIMS must integrate directly with analytical instruments, capture data automatically from spectrometers and chromatography equipment, and generate regulatory-compliant reports without manual intervention. The system should handle sample tracking across multiple locations with barcode integration that prevents mix-ups during critical testing phases.
Modern LIMS platforms process over 10,000 samples daily in major pharmaceutical facilities, with real-time status updates that researchers access through mobile devices. The platform must support electronic signatures that meet FDA requirements and maintain complete audit trails for every sample from collection through disposal. Choose systems that offer API connectivity to existing laboratory equipment rather than require expensive hardware replacements.
Manufacturing Execution Systems That Control Every Variable
Manufacturing Execution Systems control pharmaceutical production with precision that determines product quality and regulatory compliance. Your MES must monitor temperature, humidity, pressure, and mixing speeds in real-time, with automatic adjustments that maintain specifications within narrow tolerances. The system should integrate with enterprise resource planning platforms to coordinate raw material deliveries with production schedules, which prevents costly delays that disrupt batch manufacturing.
Advanced MES platforms reduce product recalls by 20-30% through predictive analytics that identify potential quality issues before they affect production batches. The system must generate batch records automatically, with electronic signatures from operators and quality control personnel at each manufacturing step. Integration with quality management systems creates seamless documentation workflows that regulatory inspectors expect during facility audits.
Quality Management Systems That Prevent Compliance Failures
Quality Management Systems serve as the central hub for all pharmaceutical compliance activities, from deviation management to corrective action tracking. Your QMS must automate document control processes, maintain version histories for all standard operating procedures, and route approvals through designated personnel based on their roles and responsibilities. The system should trigger automatic notifications when documents approach expiration dates or when quality events require immediate attention.
Modern QMS platforms handle extensive quality documentation while maintaining FDA-compliant electronic signatures and audit trails essential for regulatory inspections. The system must integrate with both LIMS and MES platforms to create a unified quality ecosystem where data flows seamlessly between laboratory results, manufacturing records, and quality documentation. This integration becomes the foundation for comprehensive cybersecurity measures that protect your most valuable pharmaceutical data and intellectual property.
How Do You Actually Protect Pharmaceutical Data
Zero-Trust Architecture for Research Protection
Pharmaceutical companies must implement zero-trust network architecture that treats every access request as potentially malicious, regardless of location or user credentials. The American Hospital Association reports that healthcare organizations faced 444 cybersecurity incidents in 2024, with 238 ransomware attacks specifically targeting patient data and research information.
Traditional perimeter security fails when intellectual property worth billions moves between research facilities, manufacturing sites, and cloud platforms. Zero-trust systems require multi-factor authentication for every data access, with continuous verification that monitors user behavior patterns and flags anomalous activities.
Network microsegmentation isolates drug discovery data from manufacturing systems, which prevents lateral movement during security breaches. Each research project needs separate network segments with role-based access controls that limit data exposure to essential personnel only.
Compliance Automation That Actually Works
Manual compliance tracking creates gaps that regulatory inspectors find during facility audits, which leads to warning letters and production delays. Automated compliance systems monitor every data modification in real-time, with electronic signatures that meet FDA 21 CFR Part 11 requirements and HIPAA privacy standards without human intervention.
These systems generate audit trails automatically and capture timestamps, user identities, and data changes across LIMS, MES, and quality management platforms. Advanced compliance automation identifies potential violations before they occur, with predictive analytics that flag unusual data access patterns or unauthorized modification attempts.
Identity Management and Access Control
The system must integrate with identity management platforms that provision and deprovision user access based on employment status and project assignments (which prevents former employees from accessing sensitive research data). Role-based access controls restrict information based on job functions, with automatic updates when personnel change positions or leave the organization.
Multi-factor authentication becomes mandatory for all system access, with biometric verification for high-security research environments. Access logs track every login attempt and data interaction, which creates comprehensive audit trails that regulatory inspectors require during facility reviews.
Final Thoughts
Pharmaceutical companies require IT solutions for pharmaceutical industry operations that address regulatory compliance automation, scalable infrastructure for multi-site research operations, and cybersecurity frameworks that protect intellectual property worth billions. The global pharmaceutical market projects ,454 billion in revenue within five years, which makes technology investments essential for competitive advantage. Partner selection requires vendors with specific pharmaceutical compliance certifications and proven experience with FDA validation processes.

Generic IT providers cannot navigate the complexities of 21 CFR Part 11 requirements or HIPAA compliance standards that pharmaceutical operations demand. Your technology partner must understand how laboratory systems integrate with manufacturing platforms and quality management workflows. Future-proof technology investments mean platforms that scale with data volumes and evolve with regulatory requirements.
AI expenditure in pharmaceuticals reaches $3 billion in 2025, while cloud adoption enables companies like AstraZeneca to analyze two million genomes with 51 billion statistical tests in under 24 hours. We at Clouddle provide cutting-edge technology solutions with managed IT, network, and security services that support complex operational requirements. Our Network as a Service combines connectivity, security, and 24/7 support without initial investment (which allows pharmaceutical companies to focus resources on drug development rather than infrastructure management).


